FLP-1
0 of 200 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
- 76
- 77
- 78
- 79
- 80
- 81
- 82
- 83
- 84
- 85
- 86
- 87
- 88
- 89
- 90
- 91
- 92
- 93
- 94
- 95
- 96
- 97
- 98
- 99
- 100
- 101
- 102
- 103
- 104
- 105
- 106
- 107
- 108
- 109
- 110
- 111
- 112
- 113
- 114
- 115
- 116
- 117
- 118
- 119
- 120
- 121
- 122
- 123
- 124
- 125
- 126
- 127
- 128
- 129
- 130
- 131
- 132
- 133
- 134
- 135
- 136
- 137
- 138
- 139
- 140
- 141
- 142
- 143
- 144
- 145
- 146
- 147
- 148
- 149
- 150
- 151
- 152
- 153
- 154
- 155
- 156
- 157
- 158
- 159
- 160
- 161
- 162
- 163
- 164
- 165
- 166
- 167
- 168
- 169
- 170
- 171
- 172
- 173
- 174
- 175
- 176
- 177
- 178
- 179
- 180
- 181
- 182
- 183
- 184
- 185
- 186
- 187
- 188
- 189
- 190
- 191
- 192
- 193
- 194
- 195
- 196
- 197
- 198
- 199
- 200
Information
SAEED MDCAT FLP SESSION 2022
PHYSICS =50
CHEMISTRY= 50
BIOLOGY= 50
ENGLISH =30
LOGICAL REASIOING =20
You have already completed the quiz before. Hence you can not start it again.
quiz is loading...
You must sign in or sign up to start the quiz.
You have to finish following quiz, to start this quiz:
Congratulations!!!" FLP-1 "
0 of 200 questions answered correctly
Time has elapsed
Your Final Score is : 0
You have attempted : 0
Number of Correct Questions : 0 and scored 0
Number of Incorrect Questions : 0 and Negative marks 0
| Average score |
|
| Your score |
|
-
Biology
You have attempted : 0
Number of Correct Questions : 0 and scored 0
Number of Incorrect Questions : 0 and Negative marks 0
-
Chemistry
You have attempted : 0
Number of Correct Questions : 0 and scored 0
Number of Incorrect Questions : 0 and Negative marks 0
-
English
You have attempted : 0
Number of Correct Questions : 0 and scored 0
Number of Incorrect Questions : 0 and Negative marks 0
-
Logical Reasoning
You have attempted : 0
Number of Correct Questions : 0 and scored 0
Number of Incorrect Questions : 0 and Negative marks 0
-
Physics
You have attempted : 0
Number of Correct Questions : 0 and scored 0
Number of Incorrect Questions : 0 and Negative marks 0
-
If you find any issue in KEY plz Inform us at 03471729745(WhatsApp)
Regard. SAEED MDCAT TEAM
HUZAIFA SAEED
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
- 76
- 77
- 78
- 79
- 80
- 81
- 82
- 83
- 84
- 85
- 86
- 87
- 88
- 89
- 90
- 91
- 92
- 93
- 94
- 95
- 96
- 97
- 98
- 99
- 100
- 101
- 102
- 103
- 104
- 105
- 106
- 107
- 108
- 109
- 110
- 111
- 112
- 113
- 114
- 115
- 116
- 117
- 118
- 119
- 120
- 121
- 122
- 123
- 124
- 125
- 126
- 127
- 128
- 129
- 130
- 131
- 132
- 133
- 134
- 135
- 136
- 137
- 138
- 139
- 140
- 141
- 142
- 143
- 144
- 145
- 146
- 147
- 148
- 149
- 150
- 151
- 152
- 153
- 154
- 155
- 156
- 157
- 158
- 159
- 160
- 161
- 162
- 163
- 164
- 165
- 166
- 167
- 168
- 169
- 170
- 171
- 172
- 173
- 174
- 175
- 176
- 177
- 178
- 179
- 180
- 181
- 182
- 183
- 184
- 185
- 186
- 187
- 188
- 189
- 190
- 191
- 192
- 193
- 194
- 195
- 196
- 197
- 198
- 199
- 200
- Answered
- Review
-
Question 1 of 200
1. Question
1 pointsCategory: PhysicsAn object is dropped from rest. Its v-t graph is
Correct
Incorrect
Unattempted
-
Question 2 of 200
2. Question
1 pointsCategory: PhysicsIf a projectile is thrown with 19.6m/s velocity at 30° with x-axis, time taken to reach highest point?
Correct
Incorrect
Unattempted
-
Question 3 of 200
3. Question
1 pointsCategory: PhysicsIf a body starts from a point, and returns back to the same point, then its
Correct
Incorrect
Unattempted
-
Question 4 of 200
4. Question
1 pointsCategory: PhysicsWhen the average velocity of a moving body is equal to its instantaneous velocity then it is moving with
Correct
Incorrect
Unattempted
-
Question 5 of 200
5. Question
1 pointsCategory: PhysicsIf the initial speed of a projectile is doubled.
Correct
Incorrect
Unattempted
-
Question 6 of 200
6. Question
1 pointsCategory: PhysicsA newton is the force
Correct
Incorrect
Unattempted
-
Question 7 of 200
7. Question
1 pointsCategory: PhysicsConsider the following five graphs (note the axes carefully). Which of these represents motion at constant speed?
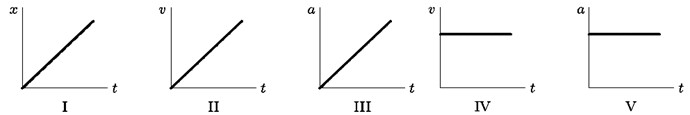 Correct
Correct
Incorrect
Unattempted
-
Question 8 of 200
8. Question
1 pointsCategory: PhysicsThe displacement time graph for a moving particle is given below. The instantaneous velocity of the particle is negative at the point.
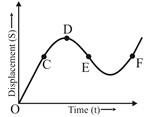 Correct
Correct
Incorrect
Unattempted
-
Question 9 of 200
9. Question
1 pointsCategory: PhysicsThe ratio of magnitudes of average velocity to average speed of a moving body in a straight line
Correct
Incorrect
Unattempted
-
Question 10 of 200
10. Question
1 pointsCategory: PhysicsThe “reaction” force does not cancel the “action” force because:
Correct
Incorrect
Unattempted
-
Question 11 of 200
11. Question
1 pointsCategory: PhysicsThe slope of v-t graph for uniform velocity is
Correct
Incorrect
Unattempted
-
Question 12 of 200
12. Question
1 pointsCategory: PhysicsNo body begin to move or comes to rest itself is statement of
Correct
Incorrect
Unattempted
-
Question 13 of 200
13. Question
1 pointsCategory: PhysicsWhen a force of 100 N acts on a body of mass 50kg then acceleration produced is
Correct
Incorrect
Unattempted
-
Question 14 of 200
14. Question
1 pointsCategory: PhysicsTime rate of change of position vector is called
Correct
Incorrect
Unattempted
-
Question 15 of 200
15. Question
1 pointsCategory: PhysicsAt maximum height in projectile motion
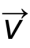 and
and 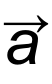 areCorrect
areCorrect
Incorrect
Unattempted
-
Question 16 of 200
16. Question
1 pointsCategory: PhysicsIf H = R then angle of projection is
Correct
Incorrect
Unattempted
-
Question 17 of 200
17. Question
1 pointsCategory: PhysicsA ball is thrown and follows a parabolic path, as shown above. Air friction is negligible. Point Q is the highest point on the path. Which of the following best indicates the direction of the acceleration, if any, of the ball at point Q?
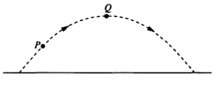 Correct
Correct
Incorrect
Unattempted
-
Question 18 of 200
18. Question
1 pointsCategory: PhysicsA force of 10 N acts on a body for 5 sec. What will be the change in momentum?
Correct
Incorrect
Unattempted
-
Question 19 of 200
19. Question
1 pointsCategory: PhysicsA particle moves through half of a circle of radius 1.0m in one second. The magnitude of average velocity is
Correct
Incorrect
Unattempted
-
Question 20 of 200
20. Question
1 pointsCategory: PhysicsA player throws a ball at an angle
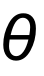 with the horizontal, the height is maximum when
with the horizontal, the height is maximum when 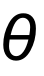 is equal toCorrect
is equal toCorrect
Incorrect
Unattempted
-
Question 21 of 200
21. Question
1 pointsCategory: PhysicsWhich graph represents the zero acceleration
Correct
Incorrect
Unattempted
-
Question 22 of 200
22. Question
1 pointsCategory: PhysicsImran travels 2m with speed v1 and then 2m with speed v2, his average speed is:
Correct
Incorrect
Unattempted
-
Question 23 of 200
23. Question
1 pointsCategory: PhysicsIf a stone is released from moving train, then the stone will follow
Correct
Incorrect
Unattempted
-
Question 24 of 200
24. Question
1 pointsCategory: PhysicsWith the help of V–t graph we can find
Correct
Incorrect
Unattempted
-
Question 25 of 200
25. Question
1 pointsCategory: PhysicsAt the highest point, the velocity of projectile is
Correct
Incorrect
Unattempted
-
Question 26 of 200
26. Question
1 pointsCategory: PhysicsThe magnitude of acceleration produced in an object is inversely proportional with
Correct
Incorrect
Unattempted
-
Question 27 of 200
27. Question
1 pointsCategory: PhysicsVelocity is defined as:
Correct
Incorrect
Unattempted
-
Question 28 of 200
28. Question
1 pointsCategory: PhysicsThe magnitude of instantaneous velocity is expressed by
Correct
Incorrect
Unattempted
-
Question 29 of 200
29. Question
1 pointsCategory: PhysicsA ball is projected at 45o its horizontal range is 10m. Velocity of projection is
Correct
Incorrect
Unattempted
-
Question 30 of 200
30. Question
1 pointsCategory: PhysicsA body, whose momentum is constant, must have constant
Correct
Incorrect
Unattempted
-
Question 31 of 200
31. Question
1 pointsCategory: PhysicsIf distance covered is zero. The displacement
Correct
Incorrect
Unattempted
-
Question 32 of 200
32. Question
1 pointsCategory: PhysicsA person can throw a stone to maximum distance of 80m the greatest height to which he can throw the stone is
Correct
Incorrect
Unattempted
-
Question 33 of 200
33. Question
1 pointsCategory: PhysicsA force of 5N acts on a body of 5kg for 5 sec. The rate of change of momentum is
Correct
Incorrect
Unattempted
-
Question 34 of 200
34. Question
1 pointsCategory: PhysicsAn aeroplane moving horizontally with 50 m/s drops a packet at 490 m height. Its time of flight is
Correct
Incorrect
Unattempted
-
Question 35 of 200
35. Question
1 pointsCategory: PhysicsA truck weighing 2500 kg and moving with a velocity of 21 m/s collides with stationary car weighing 1000 kg. The truck and the car move together after the impact. Calculate their common velocity.
Correct
Incorrect
Unattempted
-
Question 36 of 200
36. Question
1 pointsCategory: PhysicsA man is in a car is moving with velocity of 36Km/hr. his speed with respect to the car is.
Correct
Incorrect
Unattempted
-
Question 37 of 200
37. Question
1 pointsCategory: PhysicsInstantaneous and average velocities become equal when body.
Correct
Incorrect
Unattempted
-
Question 38 of 200
38. Question
1 pointsCategory: Physics3rd law of motion explains.
Correct
Incorrect
Unattempted
-
Question 39 of 200
39. Question
1 pointsCategory: PhysicsMomentum depends upon.
Correct
Incorrect
Unattempted
-
Question 40 of 200
40. Question
1 pointsCategory: PhysicsAn athlete completes one round of a circular track of radius R in 40 sec. What will be his displacement at the end of 2 min. 20 sec
Correct
Incorrect
Unattempted
-
Question 41 of 200
41. Question
1 pointsCategory: PhysicsThe ratio of the numerical values of the average velocity and average speed of a body is always
Correct
Incorrect
Unattempted
-
Question 42 of 200
42. Question
1 pointsCategory: PhysicsFrom the following displacement-time graph find out the velocity of a moving body
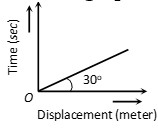 Correct
Correct
Incorrect
Unattempted
-
Question 43 of 200
43. Question
1 pointsCategory: PhysicsIn doubling the mass and acceleration of the mass, the force acting on the mass with respect to the previous value
Correct
Incorrect
Unattempted
-
Question 44 of 200
44. Question
1 pointsCategory: PhysicsK.E of a projectile at the highest point is half of its initial K.E the angle of projection is
Correct
Incorrect
Unattempted
-
Question 45 of 200
45. Question
1 pointsCategory: PhysicsA bullet is dropped from the same height when another bullet is fired horizontally. They will hit the ground
Correct
Incorrect
Unattempted
-
Question 46 of 200
46. Question
1 pointsCategory: PhysicsThe principle of conservation of linear momentum can be strictly applied during a collision between two particles provided the time of impact is
Correct
Incorrect
Unattempted
-
Question 47 of 200
47. Question
1 pointsCategory: PhysicsWhen two bodies collide elastically, then
Correct
Incorrect
Unattempted
-
Question 48 of 200
48. Question
1 pointsCategory: PhysicsWhen the speed of a moving body is doubled
Correct
Incorrect
Unattempted
-
Question 49 of 200
49. Question
1 pointsCategory: PhysicsA body of mass m collides against a wall with a velocity v and rebounds with the same speed. Its change of momentum is
Correct
Incorrect
Unattempted
-
Question 50 of 200
50. Question
1 pointsCategory: Chemistry34.2g sucrose in cup of tea have how many number of C-atoms
Correct
Incorrect
Unattempted
-
Question 51 of 200
51. Question
1 pointsCategory: Chemistry6g C react with 22414 cm3 of O2 to produce CO2. How much non-limiting reactant is in excess
Correct
Incorrect
Unattempted
-
Question 52 of 200
52. Question
1 pointsCategory: ChemistryTotal number of electrons present in 44.828 dm3 of Ne gas are
Correct
Incorrect
Unattempted
-
Question 53 of 200
53. Question
1 pointsCategory: Chemistry42g of N2 react with excess of O2 to produce NO amount of NO formed is
Correct
Incorrect
Unattempted
-
Question 54 of 200
54. Question
1 pointsCategory: ChemistryAccording to equation
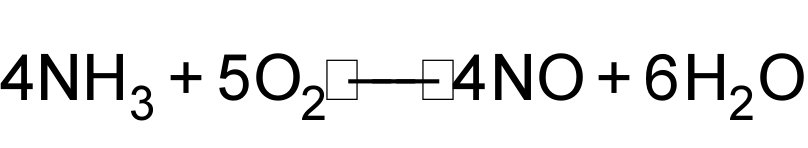 When 1mole of NH3 and 1 mol of O2 react then Correct
When 1mole of NH3 and 1 mol of O2 react then Correct
Incorrect
Unattempted
-
Question 55 of 200
55. Question
1 pointsCategory: ChemistryAccording to equation
 How many gram of H2O is formed from mixture of 51g of NH3 and 48g of oxygen Correct
How many gram of H2O is formed from mixture of 51g of NH3 and 48g of oxygen Correct
Incorrect
Unattempted
-
Question 56 of 200
56. Question
1 pointsCategory: ChemistryValue of 1 amu is equal to
Correct
Incorrect
Unattempted
-
Question 57 of 200
57. Question
1 pointsCategory: ChemistryCombustion analysis is used to determine
Correct
Incorrect
Unattempted
-
Question 58 of 200
58. Question
1 pointsCategory: ChemistryHow many moles of magnesium phosphate Mg3 (PO4) 2 will contain 0.25 mol of oxygen atoms
Correct
Incorrect
Unattempted
-
Question 59 of 200
59. Question
1 pointsCategory: ChemistryAvogadro’s number is the number of molecules present in
Correct
Incorrect
Unattempted
-
Question 60 of 200
60. Question
1 pointsCategory: ChemistryMg metal react with HCl to give hydrogen gas. What is minimum weight of HCl required to produce 6g of H2
Correct
Incorrect
Unattempted
-
Question 61 of 200
61. Question
1 pointsCategory: Chemistry31g of CuCO3 on heating give 16g of CuO. What is %age yield of reaction
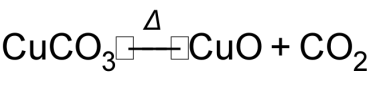 (M. mass of CuCO3 = 124gmol-1)Correct
(M. mass of CuCO3 = 124gmol-1)Correct
Incorrect
Unattempted
-
Question 62 of 200
62. Question
1 pointsCategory: ChemistryHow many molecules of NH3 would be formed if 5.6dm3 of N2 gas react with excess of H2 according to following equation
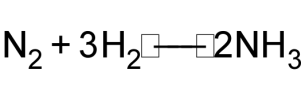 Correct
Correct
Incorrect
Unattempted
-
Question 63 of 200
63. Question
1 pointsCategory: ChemistryWhich of followings contains the maximum number of atoms
Correct
Incorrect
Unattempted
-
Question 64 of 200
64. Question
1 pointsCategory: ChemistryEmpirical formula of a hydrocarbon having 80% C and 20% of hydrogen is
Correct
Incorrect
Unattempted
-
Question 65 of 200
65. Question
1 pointsCategory: Chemistry2 mole of H2S and 11.2 dm3 of SO2 at STP react according to following equation
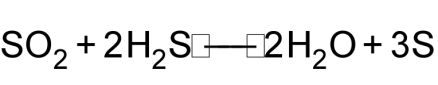 What will be number of moles of Sulphur formed in the reaction Correct
What will be number of moles of Sulphur formed in the reaction Correct
Incorrect
Unattempted
-
Question 66 of 200
66. Question
1 pointsCategory: ChemistryOne of the substance is used to absorb CO2 gas in combustion analysis. Which is that substance
Correct
Incorrect
Unattempted
-
Question 67 of 200
67. Question
1 pointsCategory: ChemistryMass of 1.505×1023 molecules of NH3 is
Correct
Incorrect
Unattempted
-
Question 68 of 200
68. Question
1 pointsCategory: ChemistryIn combustion analysis, %age of which element in always determined by difference method
Correct
Incorrect
Unattempted
-
Question 69 of 200
69. Question
1 pointsCategory: ChemistryMole may include _______ of the substance expressed in grams
Correct
Incorrect
Unattempted
-
Question 70 of 200
70. Question
1 pointsCategory: ChemistryStoichiometric calculations are made when
Correct
Incorrect
Unattempted
-
Question 71 of 200
71. Question
1 pointsCategory: ChemistryNumber of electrons in 1.7g of OH–are
Correct
Incorrect
Unattempted
-
Question 72 of 200
72. Question
1 pointsCategory: ChemistryYield which reflects the efficiency of a reaction
Correct
Incorrect
Unattempted
-
Question 73 of 200
73. Question
1 pointsCategory: ChemistryGas molecules are about _____ far away than their diameter
Correct
Incorrect
Unattempted
-
Question 74 of 200
74. Question
1 pointsCategory: ChemistryThe value of ‘n’ in determining molecular formula is obtained from the relation
Correct
Incorrect
Unattempted
-
Question 75 of 200
75. Question
1 pointsCategory: Chemistry16g of unknown gas has volume of 11.2dm3 at STP. Unknown gas is
Correct
Incorrect
Unattempted
-
Question 76 of 200
76. Question
1 pointsCategory: ChemistryFrom following equation, which relationship can be studied
 Correct
Correct
Incorrect
Unattempted
-
Question 77 of 200
77. Question
1 pointsCategory: ChemistryTheoretical yield is always less than actual yield because
Correct
Incorrect
Unattempted
-
Question 78 of 200
78. Question
1 pointsCategory: ChemistryThe molar volume of SO2 at RTP is
Correct
Incorrect
Unattempted
-
Question 79 of 200
79. Question
1 pointsCategory: ChemistryThe Avogadro’s constant is the number of
Correct
Incorrect
Unattempted
-
Question 80 of 200
80. Question
1 pointsCategory: ChemistryThe relative atomic mass of Neon is 20.18 amu. What is mass of 2 moles of Neon gas
Correct
Incorrect
Unattempted
-
Question 81 of 200
81. Question
1 pointsCategory: ChemistryWhen 1 mole of each of the following is completely burnt in oxygen. Which will give largest mass of CO2
Correct
Incorrect
Unattempted
-
Question 82 of 200
82. Question
1 pointsCategory: ChemistryAn atom of carbon is twelve times heavier than ____ atom
Correct
Incorrect
Unattempted
-
Question 83 of 200
83. Question
1 pointsCategory: ChemistryWhat is the ration of volume of 2g of hydrogen and 16g Methane? If both volumes are at STP
Correct
Incorrect
Unattempted
-
Question 84 of 200
84. Question
1 pointsCategory: Chemistry0.25 moles of phosphoric acid produces moles of H+ ions
Correct
Incorrect
Unattempted
-
Question 85 of 200
85. Question
1 pointsCategory: Chemistry18.02g of H2O sample has
Correct
Incorrect
Unattempted
-
Question 86 of 200
86. Question
1 pointsCategory: ChemistryWhich of the following sample contain maximum number of atoms?
Correct
Incorrect
Unattempted
-
Question 87 of 200
87. Question
1 pointsCategory: ChemistryConsider the following reaction involved in manufacture of ureaCO2 + 2NH3 →NH2COONH4
If 22g of CO2 react with 34g of NH3 to form ammonium carbonate, the reaction is taken as irreversible and go to completion. Identify the limiting reagent and amount of ammonium carbamate formedCorrect
Incorrect
Unattempted
-
Question 88 of 200
88. Question
1 pointsCategory: Chemistry0.1 mole of Na3PO4 completely dissociates in water to produce Na+1
Correct
Incorrect
Unattempted
-
Question 89 of 200
89. Question
1 pointsCategory: ChemistryA chemist is usually interested in
Correct
Incorrect
Unattempted
-
Question 90 of 200
90. Question
1 pointsCategory: ChemistryTotal number of electrons present in 34 g OH–are
Correct
Incorrect
Unattempted
-
Question 91 of 200
91. Question
1 pointsCategory: ChemistryMaximum number of molecules will be in
Correct
Incorrect
Unattempted
-
Question 92 of 200
92. Question
1 pointsCategory: ChemistryHow many moles of oxygen(O2) are required for complete combustion of two moles of propane (C3H)?
Correct
Incorrect
Unattempted
-
Question 93 of 200
93. Question
1 pointsCategory: Chemistry20.18 amu is the ___________ of Ne
Correct
Incorrect
Unattempted
-
Question 94 of 200
94. Question
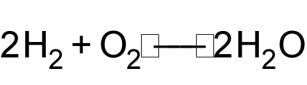
1 pointsCategory: ChemistryHydrogen reacts with oxygen as shown below 2H2(g) + O2(g)
 2H2O(l).
2H2O(l).
How much gas will remain in container if 8g of oxygen (O2) reacts with 9g of H2.Correct
Incorrect
Unattempted
-
Question 95 of 200
95. Question
1 pointsCategory: ChemistryEmpirical formula of organic compound is CH2O and molecular mass is 60g/mol. What is molecular formula
Correct
Incorrect
Unattempted
-
Question 96 of 200
96. Question
1 pointsCategory: Chemistry56g of Fe react with 24g of oxygen to produce Fe2O3. Which one is limiting reactant
Correct
Incorrect
Unattempted
-
Question 97 of 200
97. Question
1 pointsCategory: ChemistryThe largest number of molecule is present in
Correct
Incorrect
Unattempted
-
Question 98 of 200
98. Question
1 pointsCategory: ChemistryMass of one carbon atom is
Correct
Incorrect
Unattempted
-
Question 99 of 200
99. Question
1 pointsCategory: ChemistryNumber of electrons in 1.6g of methane
Correct
Incorrect
Unattempted
-
Question 100 of 200
100. Question
1 pointsCategory: BiologyIn prokaryotic cells, the cell wall is primarily made up of:
Correct
Incorrect
Unattempted
-
Question 101 of 200
101. Question
1 pointsCategory: BiologyIdentify the structure which is only found in animal cells:
Correct
Incorrect
Unattempted
-
Question 102 of 200
102. Question
1 pointsCategory: BiologyA cell has the following molecules and structures: enzymes, DNA, ribosomes, plasma membrane, andmitochondria. It could be a cell from:
Correct
Incorrect
Unattempted
-
Question 103 of 200
103. Question
1 pointsCategory: BiologyWhich of the following is true about fluid mosaic model?
Correct
Incorrect
Unattempted
-
Question 104 of 200
104. Question
1 pointsCategory: BiologyA cell organelle common in protista and monera is:
Correct
Incorrect
Unattempted
-
Question 105 of 200
105. Question
1 pointsCategory: BiologyMany cells function properly and divide mitotically even though they do not have:
Correct
Incorrect
Unattempted
-
Question 106 of 200
106. Question
1 pointsCategory: BiologyIn eukaryotic cells, nucleic acids are found in organelles other than the nucleus. Which of the following is correct?
Correct
Incorrect
Unattempted
-
Question 107 of 200
107. Question
1 pointsCategory: BiologyCell recognition and adhesion occur due to which of the following biochemicals of plasmalemma?
Correct
Incorrect
Unattempted
-
Question 108 of 200
108. Question
1 pointsCategory: BiologyWhich is near to plasma membrane?
Correct
Incorrect
Unattempted
-
Question 109 of 200
109. Question
1 pointsCategory: BiologyThe rate of diffusion of a specific substance through a plasma membrane is affected by all of the following except:
Correct
Incorrect
Unattempted
-
Question 110 of 200
110. Question
1 pointsCategory: BiologyA white blood cell is capable of producing and releasing thousands of antibody molecules every second. Since antibodies are large, complex protein molecules, then how would you expect them to leave the cell?
Correct
Incorrect
Unattempted
-
Question 111 of 200
111. Question
1 pointsCategory: BiologyPhagosomes and pinosomes are collectively referred to as:
Correct
Incorrect
Unattempted
-
Question 112 of 200
112. Question
1 pointsCategory: BiologyMiddle lamella is:
Correct
Incorrect
Unattempted
-
Question 113 of 200
113. Question
1 pointsCategory: BiologyCell wall shows:
Correct
Incorrect
Unattempted
-
Question 114 of 200
114. Question
1 pointsCategory: BiologyProtoplasmic strands between adjacent plant cells are:
Correct
Incorrect
Unattempted
-
Question 115 of 200
115. Question
1 pointsCategory: BiologyWhich of the following is not a characteristic of the cytosol?
Correct
Incorrect
Unattempted
-
Question 116 of 200
116. Question
1 pointsCategory: BiologyEnzyme that facilitates the transport through cell membrane is:
Correct
Incorrect
Unattempted
-
Question 117 of 200
117. Question
1 pointsCategory: BiologyWhat are the sac-like structures of endoplasmic reticulum?
Correct
Incorrect
Unattempted
-
Question 118 of 200
118. Question
1 pointsCategory: BiologyWhich one of the following is correctly matched?
Correct
Incorrect
Unattempted
-
Question 119 of 200
119. Question
1 pointsCategory: BiologyEndoplasmic reticulum of rapidly dividing cells is:
Correct
Incorrect
Unattempted
-
Question 120 of 200
120. Question
1 pointsCategory: BiologyPhospholipid molecules in a membrane are arranged with their ___________ on the exterior and their __________ on the interior.
Correct
Incorrect
Unattempted
-
Question 121 of 200
121. Question
1 pointsCategory: BiologyVesicles of SER are most likely on their way to:
Correct
Incorrect
Unattempted
-
Question 122 of 200
122. Question
1 pointsCategory: BiologyDictyosomes are:
Correct
Incorrect
Unattempted
-
Question 123 of 200
123. Question
1 pointsCategory: BiologyThe fluidity of membranes in a plant in cold weather may be maintained by increasing:
Correct
Incorrect
Unattempted
-
Question 124 of 200
124. Question
1 pointsCategory: BiologyBesides giving out secretory vesicles, Golgi apparatus is also concerned with formation of:
Correct
Incorrect
Unattempted
-
Question 125 of 200
125. Question
1 pointsCategory: BiologyLysosomes are so called as they have:
Correct
Incorrect
Unattempted
-
Question 126 of 200
126. Question
1 pointsCategory: BiologyWhy isn’t the mitochondrion classified as part of the endo-membrane system?
Correct
Incorrect
Unattempted
-
Question 127 of 200
127. Question
1 pointsCategory: BiologyMost hydrolytic enzymes of lysosomes function at:
Correct
Incorrect
Unattempted
-
Question 128 of 200
128. Question
1 pointsCategory: BiologyWhich of the following correctly matches an organelle with its function?
Correct
Incorrect
Unattempted
-
Question 129 of 200
129. Question
1 pointsCategory: BiologyHow do membranes function as a locus of biochemical reactions?
Correct
Incorrect
Unattempted
-
Question 130 of 200
130. Question
1 pointsCategory: BiologyThe inner membrane of mitochondria bears folding called ‘cristae’, which :
Correct
Incorrect
Unattempted
-
Question 131 of 200
131. Question
1 pointsCategory: BiologyOxysomes occur on:
Correct
Incorrect
Unattempted
-
Question 132 of 200
132. Question
1 pointsCategory: BiologyDuring oxidation, long chain fatty acids are first converted to acetyl CoA in the:
Correct
Incorrect
Unattempted
-
Question 133 of 200
133. Question
1 pointsCategory: BiologyMitochondria is a semi autonomous organelle because it contains:
Correct
Incorrect
Unattempted
-
Question 134 of 200
134. Question
1 pointsCategory: BiologyWhich of these is mismatched?
Correct
Incorrect
Unattempted
-
Question 135 of 200
135. Question
1 pointsCategory: BiologyPlastids possess:
Correct
Incorrect
Unattempted
-
Question 136 of 200
136. Question
1 pointsCategory: BiologyRibosomes are composed of:
Correct
Incorrect
Unattempted
-
Question 137 of 200
137. Question
1 pointsCategory: BiologyThe larger subunit of eukaryotic type ribosomes sediments at:
Correct
Incorrect
Unattempted
-
Question 138 of 200
138. Question
1 pointsCategory: BiologyMost of the water, in mature plant cells occurs chiefly in which of the following?
Correct
Incorrect
Unattempted
-
Question 139 of 200
139. Question
1 pointsCategory: BiologyThe nucleus is separated from surrounding cytoplasm by a nuclear membrane which is:
Correct
Incorrect
Unattempted
-
Question 140 of 200
140. Question
1 pointsCategory: BiologyA cell lacking nucleus would also lack:
Correct
Incorrect
Unattempted
-
Question 141 of 200
141. Question
1 pointsCategory: BiologyIn which method of transport in plasma membrane does not require carrier molecules?
Correct
Incorrect
Unattempted
-
Question 142 of 200
142. Question
1 pointsCategory: BiologyWhich of the following statements are correct?1. Mitochondria contain DNA
2. 70S ribosomes occur in prokaryote
3. Ribosomes are not found in Protista and Monera
4. Ribosomes are made up of phospholipids and oligosaccharidesCorrect
Incorrect
Unattempted
-
Question 143 of 200
143. Question
1 pointsCategory: BiologyMost of the intermediates in electron transport system are integral components of:
Correct
Incorrect
Unattempted
-
Question 144 of 200
144. Question
1 pointsCategory: BiologyA cell active in protein synthesis will be rich in:
Correct
Incorrect
Unattempted
-
Question 145 of 200
145. Question
1 pointsCategory: BiologyEndoplasmic reticulum is attached with:
Correct
Incorrect
Unattempted
-
Question 146 of 200
146. Question
1 pointsCategory: BiologyThe nuclear lamina is an array of filaments on the inner side of the nuclear membrane. If a method were found that could cause the lamina to fall into disarray, what would you expect to be the most likely consequence?
Correct
Incorrect
Unattempted
-
Question 147 of 200
147. Question
1 pointsCategory: BiologyWhen a lysosome fuses with phagosome, it results in the formation of:
Correct
Incorrect
Unattempted
-
Question 148 of 200
148. Question
1 pointsCategory: BiologyWhich of the following enzymes is characteristic of Golgi apparatus?
Correct
Incorrect
Unattempted
-
Question 149 of 200
149. Question
1 pointsCategory: BiologyIf nucleolus of the cell is destroyed which of these in the cell will not be formed?
Correct
Incorrect
Unattempted
-
Question 150 of 200
150. Question
1 pointsCategory: EnglishFind the Error.We thought (A) you had better looked (B) her over and tell us (C) what the matter is. (D)
Correct
Incorrect
Unattempted
-
Question 151 of 200
151. Question
1 pointsCategory: EnglishFind the Error.The farmer will (A) have watered (B) the plants by the time (C) it rained. (D)
Correct
Incorrect
Unattempted
-
Question 152 of 200
152. Question
1 pointsCategory: EnglishFind the Error.When (A) Harry got home (B), his brother was surfing (C) the web for five hours. (D)
Correct
Incorrect
Unattempted
-
Question 153 of 200
153. Question
1 pointsCategory: EnglishFind the Error.I am (A) worried (B), but (C) I daren’t to tell (D) my parents the truth.
Correct
Incorrect
Unattempted
-
Question 154 of 200
154. Question
1 pointsCategory: EnglishFind the Error.The camel man struck (A) the man himself (B) a similar blow, which (C) fell (D) him to the earth.
Correct
Incorrect
Unattempted
-
Question 155 of 200
155. Question
1 pointsCategory: EnglishFind the Error.You plan (A) to have (B) Wozzeck to pretend (C)that he has founded (D) a pearl in the oyster.
Correct
Incorrect
Unattempted
-
Question 156 of 200
156. Question
1 pointsCategory: EnglishFind the Error.It took(A) me thirty years to improve(B) these old worn-out acres to make(C) them to do (D) this.
Correct
Incorrect
Unattempted
-
Question 157 of 200
157. Question
1 pointsCategory: EnglishFind the Error.I don’t know to give (A) a poodle haircut, but (B) even if I know (C) how, I would not do (D) it.
Correct
Incorrect
Unattempted
-
Question 158 of 200
158. Question
1 pointsCategory: EnglishFind the Error.The victors killed everyone (A) on whom (B) they (C) could lie their hands. (D)
Correct
Incorrect
Unattempted
-
Question 159 of 200
159. Question
1 pointsCategory: EnglishFind the Error.It was a long time (A) before (B) she was used to(C) work (D) with old people.
Correct
Incorrect
Unattempted
-
Question 160 of 200
160. Question
1 pointsCategory: Physics1)A graph is drawn with force along Y-axis and time along X-axis. The area under the graph represents
Correct
Incorrect
Unattempted
-
Question 161 of 200
161. Question
1 pointsCategory: EnglishChoose the CORRECT option.
Correct
Incorrect
Unattempted
-
Question 162 of 200
162. Question
1 pointsCategory: EnglishChoose the CORRECT option.
Correct
Incorrect
Unattempted
-
Question 163 of 200
163. Question
1 pointsCategory: EnglishChoose the CORRECT option.
Correct
Incorrect
Unattempted
-
Question 164 of 200
164. Question
1 pointsCategory: EnglishChoose the CORRECT option.
Correct
Incorrect
Unattempted
-
Question 165 of 200
165. Question
1 pointsCategory: EnglishChoose the CORRECT option.
Correct
Incorrect
Unattempted
-
Question 166 of 200
166. Question
1 pointsCategory: EnglishChoose the CORRECT option.
Correct
Incorrect
Unattempted
-
Question 167 of 200
167. Question
1 pointsCategory: EnglishChoose the CORRECT option.
Correct
Incorrect
Unattempted
-
Question 168 of 200
168. Question
1 pointsCategory: EnglishChoose the CORRECT option.
Correct
Incorrect
Unattempted
-
Question 169 of 200
169. Question
1 pointsCategory: EnglishChoose the CORRECT option.
Correct
Incorrect
Unattempted
-
Question 170 of 200
170. Question
1 pointsCategory: EnglishChoose the CORRECT option.
Correct
Incorrect
Unattempted
-
Question 171 of 200
171. Question
1 pointsCategory: EnglishFill in the blanks.Things____________ on heating.
Correct
Incorrect
Unattempted
-
Question 172 of 200
172. Question
1 pointsCategory: EnglishFill in the blanks.Youth and age often ________ well.
Correct
Incorrect
Unattempted
-
Question 173 of 200
173. Question
1 pointsCategory: EnglishFill in the blanks.I would rather not ________ it anymore, if you don’t mind
Correct
Incorrect
Unattempted
-
Question 174 of 200
174. Question
1 pointsCategory: EnglishFill in the blanks.I _______ at a sports shop for six weeks.
Correct
Incorrect
Unattempted
-
Question 175 of 200
175. Question
1 pointsCategory: EnglishFill in the blanks.You performed better than he __________.
Correct
Incorrect
Unattempted
-
Question 176 of 200
176. Question
1 pointsCategory: EnglishFill in the blanks.Going to the dentist needn’t necessarily ______ a painful experience.
Correct
Incorrect
Unattempted
-
Question 177 of 200
177. Question
1 pointsCategory: EnglishFill in the blanks.Dare she ____him at the office?
Correct
Incorrect
Unattempted
-
Question 178 of 200
178. Question
1 pointsCategory: EnglishFill in the blanks.I was not averse to____ with any boy who challenged me.
Correct
Incorrect
Unattempted
-
Question 179 of 200
179. Question
1 pointsCategory: EnglishFill in the blanks.Would that we____ her before she died.
Correct
Incorrect
Unattempted
-
Question 180 of 200
180. Question
1 pointsCategory: EnglishFill in the blanks.Pakistani people ___ Qameeze shalwar.
Correct
Incorrect
Unattempted
-
Question 181 of 200
181. Question
1 pointsCategory: Logical ReasoningFind out the wrong term 8, 14, 26,48, 98, 194, 386
Correct
Incorrect
Unattempted
-
Question 182 of 200
182. Question
1 pointsCategory: Logical Reasoning8, 24, 12, 36, 18, 54, ?
Correct
Incorrect
Unattempted
-
Question 183 of 200
183. Question
1 pointsCategory: Logical Reasoning2, 10, 30, 68, 130, ?
Correct
Incorrect
Unattempted
-
Question 184 of 200
184. Question
1 pointsCategory: Logical ReasoningCX FU IR ? OL RI
Correct
Incorrect
Unattempted
-
Question 185 of 200
185. Question
1 pointsCategory: Logical ReasoningFlower : Butterfly :: Dirt : ?
Correct
Incorrect
Unattempted
-
Question 186 of 200
186. Question
1 pointsCategory: Logical Reasoning0, 2, 6, ?, 20, 30, 42
Correct
Incorrect
Unattempted
-
Question 187 of 200
187. Question
1 pointsCategory: Logical Reasoning3, 10, 20, 33, 49, 68, ?
Correct
Incorrect
Unattempted
-
Question 188 of 200
188. Question
1 pointsCategory: Logical ReasoningIf GIVE is coded as 5137 and BAT is coded as 924, how is GATE coded?
Correct
Incorrect
Unattempted
-
Question 189 of 200
189. Question
1 pointsCategory: Logical ReasoningP3C, R5F, T8I, V12L,?
Correct
Incorrect
Unattempted
-
Question 190 of 200
190. Question
1 pointsCategory: Logical ReasoningIf PLANE is coded as OKZMD in a certain language, how will TRAIN be coded?
Correct
Incorrect
Unattempted
-
Question 191 of 200
191. Question
1 pointsCategory: Logical ReasoningCalendar: Dates:: Dictionary : ?
Correct
Incorrect
Unattempted
-
Question 192 of 200
192. Question
1 pointsCategory: Logical Reasoning71, 76, 69, 74, 67, 72, ?
Correct
Incorrect
Unattempted
-
Question 193 of 200
193. Question
1 pointsCategory: Logical ReasoningSelect the correct pattern
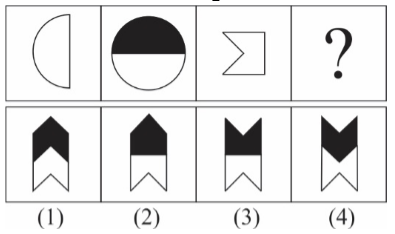 Correct
Correct
Incorrect
Unattempted
-
Question 194 of 200
194. Question
1 pointsCategory: Logical ReasoningSelect the correct pattern
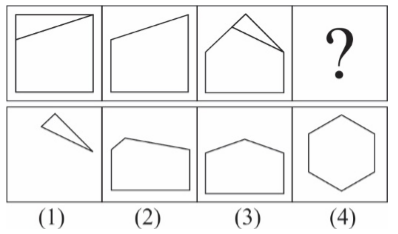 Correct
Correct
Incorrect
Unattempted
-
Question 195 of 200
195. Question
1 pointsCategory: Logical ReasoningSelect the correct pattern
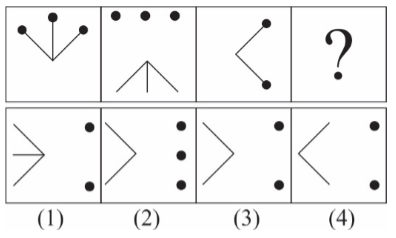 Correct
Correct
Incorrect
Unattempted
-
Question 196 of 200
196. Question
1 pointsCategory: Logical ReasoningChoose out the odd one.
Correct
Incorrect
Unattempted
-
Question 197 of 200
197. Question
1 pointsCategory: Logical ReasoningChoose out the odd one.
Correct
Incorrect
Unattempted
-
Question 198 of 200
198. Question
1 pointsCategory: Logical ReasoningCountry cannot exist without
Correct
Incorrect
Unattempted
-
Question 199 of 200
199. Question
1 pointsCategory: Logical ReasoningIf + stands for division, – stands for addition, × stands for subtraction and
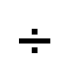 stands for multiplication then
stands for multiplication then 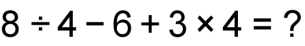 Correct
Correct
Incorrect
Unattempted
-
Question 200 of 200
200. Question
1 pointsCategory: Logical ReasoningWater : Oxygen
Correct
Incorrect
Unattempted
Leaderboard: FLP-1
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||
You are great sir thanks for uploading these tests on wesites we are preparing in amazing way
Key should also be provided so that we can mend our mistakes. Thanks 👍
Best 👍💯
Good plat form
Sach mn?
Helpful questions
Good plat form helpful for students and most important free ❤️
THANKS
How to save the test when we solved it ?
Bcz we can learn from our mistakes
Good
Good plat form
May get success🙂🙂
Great efforts thanks sir
May Allah bless you 😍
Best sir
Sir,mdcat courses me ,early prep session aur CTS show ni ho rhe,,kindly reshow krdein