FLP-06
0 of 200 questions completed
Questions:
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
- 76
- 77
- 78
- 79
- 80
- 81
- 82
- 83
- 84
- 85
- 86
- 87
- 88
- 89
- 90
- 91
- 92
- 93
- 94
- 95
- 96
- 97
- 98
- 99
- 100
- 101
- 102
- 103
- 104
- 105
- 106
- 107
- 108
- 109
- 110
- 111
- 112
- 113
- 114
- 115
- 116
- 117
- 118
- 119
- 120
- 121
- 122
- 123
- 124
- 125
- 126
- 127
- 128
- 129
- 130
- 131
- 132
- 133
- 134
- 135
- 136
- 137
- 138
- 139
- 140
- 141
- 142
- 143
- 144
- 145
- 146
- 147
- 148
- 149
- 150
- 151
- 152
- 153
- 154
- 155
- 156
- 157
- 158
- 159
- 160
- 161
- 162
- 163
- 164
- 165
- 166
- 167
- 168
- 169
- 170
- 171
- 172
- 173
- 174
- 175
- 176
- 177
- 178
- 179
- 180
- 181
- 182
- 183
- 184
- 185
- 186
- 187
- 188
- 189
- 190
- 191
- 192
- 193
- 194
- 195
- 196
- 197
- 198
- 199
- 200
Information
SAEED MDCAT FLP SESSION 2022
PHYSICS =50
CHEMISTRY= 50
BIOLOGY= 50
ENGLISH =30
LOGICAL REASIOING =20
You have already completed the quiz before. Hence you can not start it again.
quiz is loading...
You must sign in or sign up to start the quiz.
You have to finish following quiz, to start this quiz:
Congratulations!!!" FLP-06 "
0 of 200 questions answered correctly
Your time:
Time has elapsed
Your Final Score is : 0
You have attempted : 0
Number of Correct Questions : 0 and scored 0
Number of Incorrect Questions : 0 and Negative marks 0
| Average score |
|
| Your score |
|
-
Biology
You have attempted : 0
Number of Correct Questions : 0 and scored 0
Number of Incorrect Questions : 0 and Negative marks 0
-
Chemistry
You have attempted : 0
Number of Correct Questions : 0 and scored 0
Number of Incorrect Questions : 0 and Negative marks 0
-
English
You have attempted : 0
Number of Correct Questions : 0 and scored 0
Number of Incorrect Questions : 0 and Negative marks 0
-
Logical Reasoning
You have attempted : 0
Number of Correct Questions : 0 and scored 0
Number of Incorrect Questions : 0 and Negative marks 0
-
Physics
You have attempted : 0
Number of Correct Questions : 0 and scored 0
Number of Incorrect Questions : 0 and Negative marks 0
-
Discussion Video For this Session Is paid
If you want to buy ,Please Contact us at 03471729745(WhatsApp)
- 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
- 11
- 12
- 13
- 14
- 15
- 16
- 17
- 18
- 19
- 20
- 21
- 22
- 23
- 24
- 25
- 26
- 27
- 28
- 29
- 30
- 31
- 32
- 33
- 34
- 35
- 36
- 37
- 38
- 39
- 40
- 41
- 42
- 43
- 44
- 45
- 46
- 47
- 48
- 49
- 50
- 51
- 52
- 53
- 54
- 55
- 56
- 57
- 58
- 59
- 60
- 61
- 62
- 63
- 64
- 65
- 66
- 67
- 68
- 69
- 70
- 71
- 72
- 73
- 74
- 75
- 76
- 77
- 78
- 79
- 80
- 81
- 82
- 83
- 84
- 85
- 86
- 87
- 88
- 89
- 90
- 91
- 92
- 93
- 94
- 95
- 96
- 97
- 98
- 99
- 100
- 101
- 102
- 103
- 104
- 105
- 106
- 107
- 108
- 109
- 110
- 111
- 112
- 113
- 114
- 115
- 116
- 117
- 118
- 119
- 120
- 121
- 122
- 123
- 124
- 125
- 126
- 127
- 128
- 129
- 130
- 131
- 132
- 133
- 134
- 135
- 136
- 137
- 138
- 139
- 140
- 141
- 142
- 143
- 144
- 145
- 146
- 147
- 148
- 149
- 150
- 151
- 152
- 153
- 154
- 155
- 156
- 157
- 158
- 159
- 160
- 161
- 162
- 163
- 164
- 165
- 166
- 167
- 168
- 169
- 170
- 171
- 172
- 173
- 174
- 175
- 176
- 177
- 178
- 179
- 180
- 181
- 182
- 183
- 184
- 185
- 186
- 187
- 188
- 189
- 190
- 191
- 192
- 193
- 194
- 195
- 196
- 197
- 198
- 199
- 200
- Answered
- Review
-
Question 1 of 200
1. Question
1 pointsCategory: Logical ReasoningIf in a certain language GRASP is coded as BMVNK, which word would be coded as CRANE?
Correct
Incorrect
Unattempted
-
Question 2 of 200
2. Question
1 pointsCategory: Logical ReasoningStatements:
Tom puts on his socks before he puts on his shoes.
He puts on his shirt before he puts on his jacket.
Tom puts on his shoes before he puts on his shirt.
If the first two statements are true, the third statement isCorrect
Incorrect
Unattempted
-
Question 3 of 200
3. Question
1 pointsCategory: Logical ReasoningStatements:
Some sacks are backs.
All backs are bones.
No bone is muscle.
Conclusions:
I. Some sacks are not muscles.
II. Some sacks are not bones.
III. All sacks are bones.
IV. No sack is muscle.Correct
Incorrect
Unattempted
-
Question 4 of 200
4. Question
1 pointsCategory: Logical Reasoning2, 0, 5, 3, ?, 8, 17
Correct
Incorrect
Unattempted
-
Question 5 of 200
5. Question
1 pointsCategory: Logical ReasoningWhat should come next in the series 12 234 3456?
Correct
Incorrect
Unattempted
-
Question 6 of 200
6. Question
1 pointsCategory: Logical ReasoningFind the one which does not belong to that group
Correct
Incorrect
Unattempted
-
Question 7 of 200
7. Question
1 pointsCategory: PhysicsA 2 kg mass of copper is heated for 40s by a heater that produces 100J/s. The specific heat capacity of copper is 400j/(kg K). What is the rise in temperature?
Correct
Incorrect
Unattempted
-
Question 8 of 200
8. Question
1 pointsCategory: PhysicsWhich of the following is true for a closed system?
Correct
Incorrect
Unattempted
-
Question 9 of 200
9. Question
1 pointsCategory: PhysicsThe temperature of 2 mole of a gas is changed. From 100°C to l20°C at constant volume. The change in internal energy was found to be 80 J. What is the molar heat capacity of this gas at constant volume?
Correct
Incorrect
Unattempted
-
Question 10 of 200
10. Question
1 pointsCategory: PhysicsA point on p-v diagram represent
Correct
Incorrect
Unattempted
-
Question 11 of 200
11. Question
1 pointsCategory: PhysicsA gas is compressed at a constant pressure of 50 N/m2 from a volume of 10m3 to a volume of 4m3. Energy of 100 J then added to the gas by heating. Its internal energy is
Correct
Incorrect
Unattempted
-
Question 12 of 200
12. Question
1 pointsCategory: PhysicsWhen compressed gas is suddenly allowed to expand, which of the following equation determines the p – v relationship with g being the gas constant?
Correct
Incorrect
Unattempted
-
Question 13 of 200
13. Question
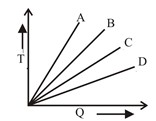
1 pointsCategory: PhysicsWhich of the substances A,B or C has the highest specific heat? The temperature Vs heat graph is
 Correct
Correct
Incorrect
Unattempted
-
Question 14 of 200
14. Question
1 pointsCategory: PhysicsA thermo-dynamical system is changed from state
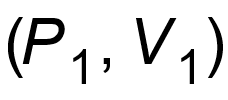 to
to 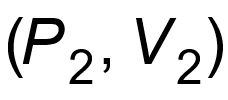 by two different process. The quantity which will remain same will beCorrect
by two different process. The quantity which will remain same will beCorrect
Incorrect
Unattempted
-
Question 15 of 200
15. Question
1 pointsCategory: PhysicsIf 1 mole of an ideal gas is heated at constant pressure, then
Correct
Incorrect
Unattempted
-
Question 16 of 200
16. Question
1 pointsCategory: PhysicsThermodynamic is the study of relationship between
Correct
Incorrect
Unattempted
-
Question 17 of 200
17. Question
1 pointsCategory: PhysicsThe curve represents isothermal process is called
Correct
Incorrect
Unattempted
-
Question 18 of 200
18. Question
1 pointsCategory: PhysicsConsider the ratios of the heat capacities γ = Cp/Cv for the three types of ideal gases: monatomic, diatomic, and polyatomic.
Correct
Incorrect
Unattempted
-
Question 19 of 200
19. Question
1 pointsCategory: PhysicsWhen two bodies are said to be in thermal equilibrium, then net exchange of heat between them is:
Correct
Incorrect
Unattempted
-
Question 20 of 200
20. Question
1 pointsCategory: PhysicsThe relation between work and heat of a system is:
Correct
Incorrect
Unattempted
-
Question 21 of 200
21. Question
1 pointsCategory: PhysicsInternal Energy of a body is maximum, when its temperature is:
Correct
Incorrect
Unattempted
-
Question 22 of 200
22. Question
1 pointsCategory: PhysicsIf a system undergoes contraction of volume, then the work done by the system will be
Correct
Incorrect
Unattempted
-
Question 23 of 200
23. Question
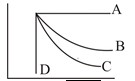
1 pointsCategory: PhysicsWhich curve shown in fig is adiabatic
 Correct
Correct
Incorrect
Unattempted
-
Question 24 of 200
24. Question
1 pointsCategory: PhysicsWhen heat in given to a gas in an isobaric process, then
Correct
Incorrect
Unattempted
-
Question 25 of 200
25. Question
1 pointsCategory: PhysicsWhich of the following is correct in terms of increasing work done for the same initial and final state?
Correct
Incorrect
Unattempted
-
Question 26 of 200
26. Question
1 pointsCategory: PhysicsAs
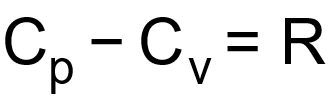 shows that
shows that 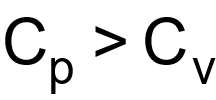 . What is also true?Correct
. What is also true?Correct
Incorrect
Unattempted
-
Question 27 of 200
27. Question
1 pointsCategory: PhysicsBy rubbing the objects together, their internal energy:
Correct
Incorrect
Unattempted
-
Question 28 of 200
28. Question
1 pointsCategory: PhysicsThe rapid expansion and compression of air through which a sound wave is passing, obeys
Correct
Incorrect
Unattempted
-
Question 29 of 200
29. Question
1 pointsCategory: PhysicsThe change in internal energy by the system in going through cycle is
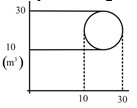 Correct
Correct
Incorrect
Unattempted
-
Question 30 of 200
30. Question
1 pointsCategory: Physics110 J of heat is added to a gaseous system, whose internal energy change is 40 J, then the amount of external work done is
Correct
Incorrect
Unattempted
-
Question 31 of 200
31. Question
1 pointsCategory: PhysicsWhich one of the following process has greater slope in P – V diagram; (Take, V, along x-axis and ‘P’ along y-axis)
Correct
Incorrect
Unattempted
-
Question 32 of 200
32. Question
1 pointsCategory: PhysicsIn an adiabatic process,
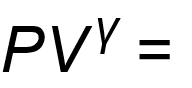 constant; the value ‘
constant; the value ‘ ’ isCorrect
’ isCorrect
Incorrect
Unattempted
-
Question 33 of 200
33. Question
1 pointsCategory: Physics50 J heat is transferred slowly to a gas which pushes the piston of cross-sectional area 0.2 m2 through a distance of 2.0 cm and pressure of the gas is maintained at 10000 Nm -2. Change in internal energy of gas during expansion is
Correct
Incorrect
Unattempted
-
Question 34 of 200
34. Question
1 pointsCategory: PhysicsConsider boiling water converting into steam. Under this condition, the specific heat of water is
Correct
Incorrect
Unattempted
-
Question 35 of 200
35. Question
1 pointsCategory: PhysicsFirst law of thermodynamics is applicable for
Correct
Incorrect
Unattempted
-
Question 36 of 200
36. Question
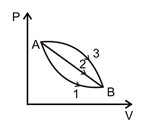
1 pointsCategory: PhysicsA given mass of a gas expands from state A to the state B by three paths 1, 2 and 3 as shown in the figure. If W1, W2 and W3 respectively be the work done by the gas along three paths then
 Correct
Correct
Incorrect
Unattempted
-
Question 37 of 200
37. Question
1 pointsCategory: PhysicsDuring an adiabatic expansion the increase in volume is associated with:
Correct
Incorrect
Unattempted
-
Question 38 of 200
38. Question
1 pointsCategory: PhysicsDuring an adiabatic expansion of 5 moles of gas, the internal energy decreases by –75J. The work done during the process is
Correct
Incorrect
Unattempted
-
Question 39 of 200
39. Question
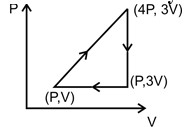
1 pointsCategory: PhysicsAn ideal monatomic gas has taken round the cycle, work done during the cycle is:
 Correct
Correct
Incorrect
Unattempted
-
Question 40 of 200
40. Question
1 pointsCategory: PhysicsWhat can be calculated from the curve under PV graph
Correct
Incorrect
Unattempted
-
Question 41 of 200
41. Question
1 pointsCategory: PhysicsHeat given to an ideal gas under isothermal conditions is used
Correct
Incorrect
Unattempted
-
Question 42 of 200
42. Question
1 pointsCategory: PhysicsWe consider a thermodynamic system. If
 U represents the increase in its energy and W the work done by the system, which of the following statements is true?Correct
U represents the increase in its energy and W the work done by the system, which of the following statements is true?Correct
Incorrect
Unattempted
-
Question 43 of 200
43. Question
1 pointsCategory: PhysicsA system does 600J of work at the same time has its internal energy increased by 320J. How much heat has been supplied?
Correct
Incorrect
Unattempted
-
Question 44 of 200
44. Question
1 pointsCategory: PhysicsThe internal energy of a monoatomic ideal gas is
Correct
Incorrect
Unattempted
-
Question 45 of 200
45. Question
1 pointsCategory: PhysicsInternal energy is a unique function of state because change in internal energy.
Correct
Incorrect
Unattempted
-
Question 46 of 200
46. Question
1 pointsCategory: PhysicsThe internal energy of piece of lead when beaten by a hammer will.
Correct
Incorrect
Unattempted
-
Question 47 of 200
47. Question
1 pointsCategory: PhysicsIf CP and CV are the molar specific heats of a gas at constant pressure and volume respectively then the ratio of adiabatic and isothermal moduli of elasticity will be:
Correct
Incorrect
Unattempted
-
Question 48 of 200
48. Question
1 pointsCategory: PhysicsA cycle tyre bursts suddenly. This represents an
Correct
Incorrect
Unattempted
-
Question 49 of 200
49. Question
1 pointsCategory: PhysicsA gas is being compressed adiabatically. The specific heat of the gas during compression is
Correct
Incorrect
Unattempted
-
Question 50 of 200
50. Question
1 pointsCategory: PhysicsSuppose volume of gas in a cylinder is 3 cm 3, if the piston is kept fixed and gas is heated from 5oC to 12oC then the work done is
Correct
Incorrect
Unattempted
-
Question 51 of 200
51. Question
1 pointsCategory: PhysicsIf
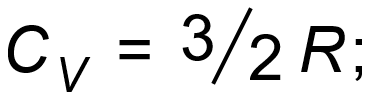 then what is
then what is 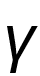 ?Correct
?Correct
Incorrect
Unattempted
-
Question 52 of 200
52. Question
1 pointsCategory: PhysicsFor an ideal gas the inter particle interaction is
Correct
Incorrect
Unattempted
-
Question 53 of 200
53. Question
1 pointsCategory: PhysicsA system undergoes an adiabatic process in which its internal energy increases by 20 J. Which of the following statements is true?
Correct
Incorrect
Unattempted
-
Question 54 of 200
54. Question
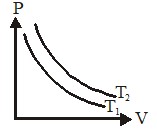
1 pointsCategory: PhysicsFigure shows the pressure P versus volume V graphs for a certain mass of a gas at two constant temperatures T1 and T2. Which of the following inferences is correct?
 Correct
Correct
Incorrect
Unattempted
-
Question 55 of 200
55. Question
1 pointsCategory: PhysicsThe energy transforming processes that occur within an organism are named as
Correct
Incorrect
Unattempted
-
Question 56 of 200
56. Question
1 pointsCategory: PhysicsFor the Boyle’s law to hold good, the necessary condition is:
Correct
Incorrect
Unattempted
-
Question 57 of 200
57. Question
1 pointsCategory: ChemistryConsider the following reaction
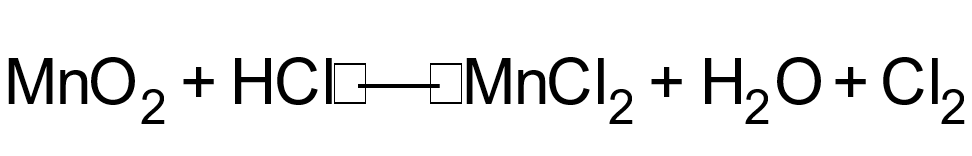 Which atom undergoes decrease in oxidation numberCorrect
Which atom undergoes decrease in oxidation numberCorrect
Incorrect
Unattempted
-
Question 58 of 200
58. Question
1 pointsCategory: ChemistryAn enthalpy change which always proceed through evolution of heat
Correct
Incorrect
Unattempted
-
Question 59 of 200
59. Question
1 pointsCategory: ChemistryAll of the following proceeds through the oxidation except
Correct
Incorrect
Unattempted
-
Question 60 of 200
60. Question
1 pointsCategory: ChemistryWhich of the following have high reduction potential then hydrogen
Correct
Incorrect
Unattempted
-
Question 61 of 200
61. Question
1 pointsCategory: ChemistryWhich of the following change in enthalpy in Born-Haber cycle may be negative
Correct
Incorrect
Unattempted
-
Question 62 of 200
62. Question
1 pointsCategory: ChemistryIn the cell shown below, which of the following is (are) true?
I. Electrons flow through the meter from left to right
II. Cu is the anode
III. The spontaneous reaction is
Cu2++ Zn → Cu + Zn2+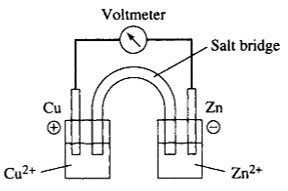 Correct
Correct
Incorrect
Unattempted
-
Question 63 of 200
63. Question
1 pointsCategory: ChemistryThe net heat change in a chemical reaction is same whether it is brought in two or more different ways in one or several steps. It is known as
Correct
Incorrect
Unattempted
-
Question 64 of 200
64. Question
1 pointsCategory: ChemistrySolutions containing chlorate (I) ions are used as household bleaches and disinfectants. These solutions decompose on heating as shown 3ClO– →ClO3–+ 2Cl– Which of the following ion will have highest oxidation state
Correct
Incorrect
Unattempted
-
Question 65 of 200
65. Question
1 pointsCategory: ChemistryWhich of the following is a list of metals in order from strongest to weakest reducing agents
Correct
Incorrect
Unattempted
-
Question 66 of 200
66. Question
1 pointsCategory: ChemistryWhich of the following will be collected at cathode during electrolysis of aq. CuSO4 solution
Correct
Incorrect
Unattempted
-
Question 67 of 200
67. Question
1 pointsCategory: ChemistryWhich of the following is incorrect about enthalpy of neutralization
Correct
Incorrect
Unattempted
-
Question 68 of 200
68. Question
1 pointsCategory: ChemistryIn exothermic reactions, the heat contents of the
Correct
Incorrect
Unattempted
-
Question 69 of 200
69. Question
1 pointsCategory: ChemistryWork may be defined in terms of pressure-volume as
Correct
Incorrect
Unattempted
-
Question 70 of 200
70. Question
1 pointsCategory: ChemistryThe condition for standard enthalpy change is
Correct
Incorrect
Unattempted
-
Question 71 of 200
71. Question
1 pointsCategory: ChemistryIf electricity is passed through CuSO4 solution by using Pt electrode, then Colour of the solution becomes fade due to
Correct
Incorrect
Unattempted
-
Question 72 of 200
72. Question
1 pointsCategory: ChemistryStronger the reducing agent greater is the
Correct
Incorrect
Unattempted
-
Question 73 of 200
73. Question
1 pointsCategory: ChemistryWhich of the following is incorrect statement about electrolytic cell
Correct
Incorrect
Unattempted
-
Question 74 of 200
74. Question
1 pointsCategory: ChemistryEored of an element can be calculated by comparing it with
Correct
Incorrect
Unattempted
-
Question 75 of 200
75. Question
1 pointsCategory: Chemistry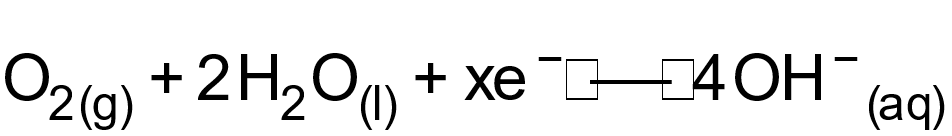 The ‘x’ electrons in above equation areCorrect
The ‘x’ electrons in above equation areCorrect
Incorrect
Unattempted
-
Question 76 of 200
76. Question
1 pointsCategory: ChemistryWhich of the following value of standard reduction potential suggest that the rate of oxidation is equal to rate of reduction
Correct
Incorrect
Unattempted
-
Question 77 of 200
77. Question
1 pointsCategory: ChemistryBorn-Haber cycle is used to determine the lattice energy of
Correct
Incorrect
Unattempted
-
Question 78 of 200
78. Question
1 pointsCategory: Chemistryif 8g of methane is burned in excess of oxygen then standard enthalpy of combustion is
Correct
Incorrect
Unattempted
-
Question 79 of 200
79. Question
1 pointsCategory: ChemistryThe system becomes more stable if it
Correct
Incorrect
Unattempted
-
Question 80 of 200
80. Question
1 pointsCategory: ChemistryWhich of the following is correct relationship at constant pressure
Correct
Incorrect
Unattempted
-
Question 81 of 200
81. Question
1 pointsCategory: ChemistryWhich of the following is correct representation for calculation of lattice energy
Correct
Incorrect
Unattempted
-
Question 82 of 200
82. Question
1 pointsCategory: ChemistryStandard reduction electrode potentials of three metals P, Q and R are +0.5 V, -3.0 V and -1.2 V respectively. The order of reducing power of these metals is
Correct
Incorrect
Unattempted
-
Question 83 of 200
83. Question
1 pointsCategory: ChemistryWhen HNO3 acts as an oxidizing agent and ultimately forms NO2, NO, HNO2 then the number of electrons transferred in each case is
Correct
Incorrect
Unattempted
-
Question 84 of 200
84. Question
1 pointsCategory: ChemistryIncorrect statement about Galvanic cell
Correct
Incorrect
Unattempted
-
Question 85 of 200
85. Question
1 pointsCategory: ChemistryAmong the following molecules, in which does Phosphorous show the lowest oxidation number
Correct
Incorrect
Unattempted
-
Question 86 of 200
86. Question
1 pointsCategory: ChemistryIn the reaction 4Al + 3O2 → 4Al3+ + 6O2– . Which of the following statements is incorrect
Correct
Incorrect
Unattempted
-
Question 87 of 200
87. Question
1 pointsCategory: ChemistryIf strip of Cu metal is placed in solution of FeSO4
Correct
Incorrect
Unattempted
-
Question 88 of 200
88. Question
1 pointsCategory: ChemistryThe reaction which proceeds through evolution of heat
Correct
Incorrect
Unattempted
-
Question 89 of 200
89. Question
1 pointsCategory: ChemistryAccording to first law of thermodynamics energy from system to surrounding can be transferred in the form of _______
Correct
Incorrect
Unattempted
-
Question 90 of 200
90. Question
1 pointsCategory: ChemistryTotal Heat content of the system is
Correct
Incorrect
Unattempted
-
Question 91 of 200
91. Question
1 pointsCategory: ChemistryBoiling of water is __________ change
Correct
Incorrect
Unattempted
-
Question 92 of 200
92. Question
1 pointsCategory: ChemistryThe expression
 E = q + w is Correct
E = q + w is Correct
Incorrect
Unattempted
-
Question 93 of 200
93. Question
1 pointsCategory: ChemistryIn acidic medium, moles of H2O and H + are added respectively for given reaction are
10Cl–+ 2MnO4– 2Mn2+ + 5ClCorrect
+ 5ClCorrect
Incorrect
Unattempted
-
Question 94 of 200
94. Question
1 pointsCategory: ChemistryOne Joule is equal to
Correct
Incorrect
Unattempted
-
Question 95 of 200
95. Question
1 pointsCategory: ChemistryIn a reaction, the oxidation number of Cu decreases by 2. This indicates that Cu is
Correct
Incorrect
Unattempted
-
Question 96 of 200
96. Question
1 pointsCategory: ChemistryWhen a Zn piece is placed in CuSO4 solution, copper gets precipitated because
Correct
Incorrect
Unattempted
-
Question 97 of 200
97. Question
1 pointsCategory: ChemistryAll of the following processes in Born-Haber cycle may proceed by absorption of heat except
Correct
Incorrect
Unattempted
-
Question 98 of 200
98. Question
1 pointsCategory: ChemistryDuring balancing of Redox equations by oxidation number method the ‘O’ and H-atoms are balanced by
Correct
Incorrect
Unattempted
-
Question 99 of 200
99. Question
1 pointsCategory: ChemistryWhich of the following is incorrect about enthalpy of neutralization?
Correct
Incorrect
Unattempted
-
Question 100 of 200
100. Question
1 pointsCategory: ChemistryStandard enthalpy of Al 2O3 cannot be measured because
Correct
Incorrect
Unattempted
-
Question 101 of 200
101. Question
1 pointsCategory: ChemistryThe dissolution of ammonium chloride in water is a/an
Correct
Incorrect
Unattempted
-
Question 102 of 200
102. Question
1 pointsCategory: Chemistry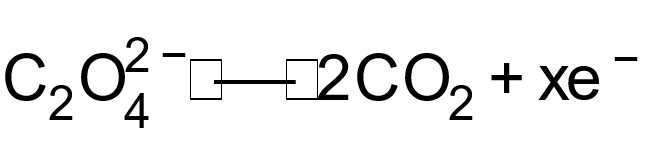
The ‘x’ electrons in above equation areCorrect
Incorrect
Unattempted
-
Question 103 of 200
103. Question
1 pointsCategory: ChemistryIf enthalpy of product is more than reactant the value of
 H is denoted byCorrect
H is denoted byCorrect
Incorrect
Unattempted
-
Question 104 of 200
104. Question
1 pointsCategory: ChemistryWhich is used to measure enthalpy of combustion
Correct
Incorrect
Unattempted
-
Question 105 of 200
105. Question
1 pointsCategory: ChemistryIf number of moles of product is more than that of reactant than
Correct
Incorrect
Unattempted
-
Question 106 of 200
106. Question
1 pointsCategory: ChemistryThe amount of heat required to convert one mole of a solid directly into its vapour state at STP is called as
Correct
Incorrect
Unattempted
-
Question 107 of 200
107. Question
1 pointsCategory: BiologyAmong the following characteristics, which one is unique to animals?
Correct
Incorrect
Unattempted
-
Question 108 of 200
108. Question
1 pointsCategory: BiologyBoth animals and fungi are heterotrophic. What distinguishes animal heterotrophy from fungal heterotrophy is that only animals derive their nutrition:
Correct
Incorrect
Unattempted
-
Question 109 of 200
109. Question
1 pointsCategory: BiologyThe grade of organization in sponges is:
Correct
Incorrect
Unattempted
-
Question 110 of 200
110. Question
1 pointsCategory: BiologyWhich level of organization is found in majority of animals?
Correct
Incorrect
Unattempted
-
Question 111 of 200
111. Question
1 pointsCategory: BiologySac like digestive system is shown by:
Correct
Incorrect
Unattempted
-
Question 112 of 200
112. Question
1 pointsCategory: BiologyRadial symmetry occurs in animals having:
Correct
Incorrect
Unattempted
-
Question 113 of 200
113. Question
1 pointsCategory: BiologyAnimals with radial symmetry in adult and bilateral symmetry in larvae are:
Correct
Incorrect
Unattempted
-
Question 114 of 200
114. Question
1 pointsCategory: BiologyWhich of the following is a correct association of an animal germ layer with the tissues or organs to which it gives rise?
Correct
Incorrect
Unattempted
-
Question 115 of 200
115. Question
1 pointsCategory: BiologyThe following animal phylum belongs to Deuterostomia:
Correct
Incorrect
Unattempted
-
Question 116 of 200
116. Question
1 pointsCategory: BiologyAll diploblastic animals are:
Correct
Incorrect
Unattempted
-
Question 117 of 200
117. Question
1 pointsCategory: BiologyA coelom (body cavity) derived from blastocoels is known as:
Correct
Incorrect
Unattempted
-
Question 118 of 200
118. Question
1 pointsCategory: BiologyCoelom is a space between:
Correct
Incorrect
Unattempted
-
Question 119 of 200
119. Question
1 pointsCategory: BiologyThe following animal phylum belongs to Deuterostomia:
Correct
Incorrect
Unattempted
-
Question 120 of 200
120. Question
1 pointsCategory: BiologyWhat is characteristic of deuterostomes?
Correct
Incorrect
Unattempted
-
Question 121 of 200
121. Question
1 pointsCategory: BiologyThe spongocoel of the sponge is lined with:
Correct
Incorrect
Unattempted
-
Question 122 of 200
122. Question
1 pointsCategory: BiologyA pseudocoel is found in:
Correct
Incorrect
Unattempted
-
Question 123 of 200
123. Question
1 pointsCategory: BiologyWhat is the special nature of phylum porifera?
Correct
Incorrect
Unattempted
-
Question 124 of 200
124. Question
1 pointsCategory: BiologyTriploblastic, un-segmented, acoelomates exhibiting bilateral symmetry and reproducing both asexually and sexually, with some parasitic forms. The above description is characteristic of the phylum:
Correct
Incorrect
Unattempted
-
Question 125 of 200
125. Question
1 pointsCategory: BiologyCoelenterates differ from other animals in having:
Correct
Incorrect
Unattempted
-
Question 126 of 200
126. Question
1 pointsCategory: BiologyNematocysts take part in:
Correct
Incorrect
Unattempted
-
Question 127 of 200
127. Question
1 pointsCategory: BiologyCorals belong to the phylum:
Correct
Incorrect
Unattempted
-
Question 128 of 200
128. Question
1 pointsCategory: BiologyParasitic flatworms have flame cells for:
Correct
Incorrect
Unattempted
-
Question 129 of 200
129. Question
1 pointsCategory: BiologyWhich of the following is a free living flatworm?
Correct
Incorrect
Unattempted
-
Question 130 of 200
130. Question
1 pointsCategory: BiologyThe primary host of Fasciola hepatica is:
Correct
Incorrect
Unattempted
-
Question 131 of 200
131. Question
1 pointsCategory: BiologyAscaris is found in:
Correct
Incorrect
Unattempted
-
Question 132 of 200
132. Question
1 pointsCategory: BiologyEnterobius infection occurs through:
Correct
Incorrect
Unattempted
-
Question 133 of 200
133. Question
1 pointsCategory: BiologyIn evolution, ____________ evolved first time in annelids.
Correct
Incorrect
Unattempted
-
Question 134 of 200
134. Question
1 pointsCategory: BiologyMetamerism is characteristic of phylum:
Correct
Incorrect
Unattempted
-
Question 135 of 200
135. Question
1 pointsCategory: BiologyMetanephridia in annelids function as:
Correct
Incorrect
Unattempted
-
Question 136 of 200
136. Question
1 pointsCategory: BiologyMaximum diversity is found in which phylum?
Correct
Incorrect
Unattempted
-
Question 137 of 200
137. Question
1 pointsCategory: BiologyThe phylum Arthropoda is characterized by:
Correct
Incorrect
Unattempted
-
Question 138 of 200
138. Question
1 pointsCategory: BiologyExoskeleton of which of the following consists of a chitinous cuticle?
Correct
Incorrect
Unattempted
-
Question 139 of 200
139. Question
1 pointsCategory: BiologyMain excretory product in cockroach and other insects is:
Correct
Incorrect
Unattempted
-
Question 140 of 200
140. Question
1 pointsCategory: BiologyAn important pollinator is:
Correct
Incorrect
Unattempted
-
Question 141 of 200
141. Question
1 pointsCategory: BiologyThe undifferentiated layer present between the ectoderm and endoderm in coelenterate is
Correct
Incorrect
Unattempted
-
Question 142 of 200
142. Question
1 pointsCategory: BiologyWater path in sponges is
Correct
Incorrect
Unattempted
-
Question 143 of 200
143. Question
1 pointsCategory: BiologyThe character possessed by all sponges are
Correct
Incorrect
Unattempted
-
Question 144 of 200
144. Question
1 pointsCategory: BiologyPlatyhelminthes are called flat worms because
Correct
Incorrect
Unattempted
-
Question 145 of 200
145. Question
1 pointsCategory: BiologyWhich of the following is correct about parapodia?
Correct
Incorrect
Unattempted
-
Question 146 of 200
146. Question
1 pointsCategory: BiologyWhich of the following is the largest phylum?
Correct
Incorrect
Unattempted
-
Question 147 of 200
147. Question
1 pointsCategory: BiologyThe mouth which has file-like rasping organ for feeding called radula is found in:
Correct
Incorrect
Unattempted
-
Question 148 of 200
148. Question
1 pointsCategory: BiologyAnimals having calcareous endoskeleton with organ-system level of organization, completely marine are:
Correct
Incorrect
Unattempted
-
Question 149 of 200
149. Question
1 pointsCategory: BiologySea urchin belongs to the class phylum:
Correct
Incorrect
Unattempted
-
Question 150 of 200
150. Question
1 pointsCategory: BiologyFirst class of vertebrate, which are fully adopted for terrestrial life is:
Correct
Incorrect
Unattempted
-
Question 151 of 200
151. Question
1 pointsCategory: BiologyWhich of the following assists in the locomotion of the organism stated?
Correct
Incorrect
Unattempted
-
Question 152 of 200
152. Question
1 pointsCategory: BiologyA terrestrial animal must be able to:
Correct
Incorrect
Unattempted
-
Question 153 of 200
153. Question
1 pointsCategory: BiologyPresence of gills in the tadpole of frog indicates that:
Correct
Incorrect
Unattempted
-
Question 154 of 200
154. Question
1 pointsCategory: BiologyNutritionally hydra is:
Correct
Incorrect
Unattempted
-
Question 155 of 200
155. Question
1 pointsCategory: BiologyA wood boring mollusk, well known form causing damage to ships and docks is:
Correct
Incorrect
Unattempted
-
Question 156 of 200
156. Question
1 pointsCategory: BiologyWhich cells in a sponge are primarily responsible for trapping and removing food particles from circulating water?
Correct
Incorrect
Unattempted
-
Question 157 of 200
157. Question
1 pointsCategory: EnglishFind the Error.This car is too much (A) expensive for me (B) just to think of it, (C) let alone buy it. (D)
Correct
Incorrect
Unattempted
-
Question 158 of 200
158. Question
1 pointsCategory: EnglishFind the Error.Despite the risks, (A) most experts think (B) that shares will do better (C) than other assets. (D)
Correct
Incorrect
Unattempted
-
Question 159 of 200
159. Question
1 pointsCategory: EnglishFind the Error.Mr. Smith is the most suitable person (A) for such cases (B) since his experience (C) and quickness of mind are equal to the occasion. (D)
Correct
Incorrect
Unattempted
-
Question 160 of 200
160. Question
1 pointsCategory: EnglishFind the Error.It is important that (A) she appreciate (B) you care for (C) the homeless and the needy. (D)
Correct
Incorrect
Unattempted
-
Question 161 of 200
161. Question
1 pointsCategory: EnglishFind the Error.Hamza is the eagerest student (A) Ms. Johnson has, (B) and she has great expectations (C) of him. (D)
Correct
Incorrect
Unattempted
-
Question 162 of 200
162. Question
1 pointsCategory: EnglishFind the Error.It goes without saying (A) that the interest (B) at a loan company (C) is higher than a bank. (D)
Correct
Incorrect
Unattempted
-
Question 163 of 200
163. Question
1 pointsCategory: EnglishFind the Error.Athletes can be just as unhealthy, (A) or even unhealthier than, (B) people who lead (C) sedentary lifestyles. (D)
Correct
Incorrect
Unattempted
-
Question 164 of 200
164. Question
1 pointsCategory: EnglishFind the Error.Our last prophet (A) (peace be upon him) is the most perfect example (B) of morality (C) and character. (D)
Correct
Incorrect
Unattempted
-
Question 165 of 200
165. Question
1 pointsCategory: EnglishFind the Error.Most people think (A) it is harder to understand (B) Freud’s theory (C) than Watson. (D)
Correct
Incorrect
Unattempted
-
Question 166 of 200
166. Question
1 pointsCategory: EnglishFind the Error.On neither of those trips (A) was there (B) sun enough (C) to get any decent photographs. (D)
Correct
Incorrect
Unattempted
-
Question 167 of 200
167. Question
1 pointsCategory: EnglishFind the Error.I felt badly (A) about not being able (B) to come to you (C) to bid you a final farewell. (D)
Correct
Incorrect
Unattempted
-
Question 168 of 200
168. Question
1 pointsCategory: EnglishFind the Error.The aircraft (A) caught fire (B) and put the crew (C) in a high dangerous situation. (D)
Correct
Incorrect
Unattempted
-
Question 169 of 200
169. Question
1 pointsCategory: EnglishFind the Error.Nadia is a novelist, essayist, (A) playwright, and poet (B) ,moreover, (C) she is a distinguished scholar. (D)
Correct
Incorrect
Unattempted
-
Question 170 of 200
170. Question
1 pointsCategory: EnglishFill in the blanks.I think England will win, but _______ people agree with me.
Correct
Incorrect
Unattempted
-
Question 171 of 200
171. Question
1 pointsCategory: EnglishFill in the blanks.I don’t have _______ money left.
Correct
Incorrect
Unattempted
-
Question 172 of 200
172. Question
1 pointsCategory: EnglishFill in the blanks.We need _______ furniture in this office than in the big one.
Correct
Incorrect
Unattempted
-
Question 173 of 200
173. Question
1 pointsCategory: EnglishFill in the blanks.Aisha is _______ of the two candidates.
Correct
Incorrect
Unattempted
-
Question 174 of 200
174. Question
1 pointsCategory: EnglishFill in the blanks.Tom and Dick were both heroes, but only the _______ is remembered today.
Correct
Incorrect
Unattempted
-
Question 175 of 200
175. Question
1 pointsCategory: EnglishFill in the blanks.He behaved towards them _______.
Correct
Incorrect
Unattempted
-
Question 176 of 200
176. Question
1 pointsCategory: EnglishFill in the blanks.She was _______ who didn’t care for the poor.
Correct
Incorrect
Unattempted
-
Question 177 of 200
177. Question
1 pointsCategory: EnglishIn each of the following questions, four alternative sentences are given. Choose the correct one.
Correct
Incorrect
Unattempted
-
Question 178 of 200
178. Question
1 pointsCategory: EnglishIn each of the following questions, four alternative sentences are given. Choose the correct one.
Correct
Incorrect
Unattempted
-
Question 179 of 200
179. Question
1 pointsCategory: EnglishIn each of the following questions, four alternative sentences are given. Choose the correct one.
Correct
Incorrect
Unattempted
-
Question 180 of 200
180. Question
1 pointsCategory: EnglishIn each of the following questions, four alternative sentences are given. Choose the correct one.
Correct
Incorrect
Unattempted
-
Question 181 of 200
181. Question
1 pointsCategory: EnglishIn each of the following questions, four alternative sentences are given. Choose the correct one.
Correct
Incorrect
Unattempted
-
Question 182 of 200
182. Question
1 pointsCategory: EnglishIn each of the following questions, four alternative sentences are given. Choose the correct one.
Correct
Incorrect
Unattempted
-
Question 183 of 200
183. Question
1 pointsCategory: EnglishIn each of the following questions, four alternative sentences are given. Choose the correct one.
Correct
Incorrect
Unattempted
-
Question 184 of 200
184. Question
1 pointsCategory: EnglishIn each of the following questions, four alternative sentences are given. Choose the correct one.
Correct
Incorrect
Unattempted
-
Question 185 of 200
185. Question
1 pointsCategory: EnglishIn each of the following questions, four alternative sentences are given. Choose the correct one.
Correct
Incorrect
Unattempted
-
Question 186 of 200
186. Question
1 pointsCategory: EnglishIn each of the following questions, four alternative sentences are given. Choose the correct one.
Correct
Incorrect
Unattempted
-
Question 187 of 200
187. Question
1 pointsCategory: Logical ReasoningStatement:
A lot of unscrupulous and non affiliated colleges have started to lure unsuspecting students by giving attractive advertisements
Courses of Action
I. Students should make appropriate enquiries while enrolling in any course.
II. The government should initiate strict action against such college authorities.Correct
Incorrect
Unattempted
-
Question 188 of 200
188. Question
1 pointsCategory: Logical ReasoningStatement:
The colony has suffered major thefts and break-ins due to lax security systems
Courses of Action
I. Security should be strengthened in the colony.
II. Residents and regular visitors and their vehicles should be provided with identity cards and stickers for better control of who is coming and going into the colony.Correct
Incorrect
Unattempted
-
Question 189 of 200
189. Question
1 pointsCategory: Logical ReasoningStatement:
The LESCO has been unable to provide 24 hours electricity leading to tremendous economic loss.
Courses of Action
I. The Government must provide for increasing electricity consumption
II. The government should check the electricity theft cases.Correct
Incorrect
Unattempted
-
Question 190 of 200
190. Question
1 pointsCategory: Logical ReasoningStatement:
A sting operation conducted by a TV news channel proved to be a total failure as the reporter who carried out the sting was found to have created a fake sting in order to gain publicity and money.
Courses of Action
I. Disciplinary action must be initiated immediately against the reporter.
II. The TV channel should be penalized and taken off air for a short period of time.Correct
Incorrect
Unattempted
-
Question 191 of 200
191. Question
1 pointsCategory: Logical ReasoningStatement:
There is a shortage of power in Pakistan.
Courses of Action
I. There should be more power projects initiated by the government.
II. The government should encourage private investment in power projects.Correct
Incorrect
Unattempted
-
Question 192 of 200
192. Question
1 pointsCategory: Logical ReasoningStatement:
The reduction of the tax rates has led to an increase in the tax collection as there has been higher compliance
Courses of Action
I. It should be made compulsory every Pakistani to pay tax.
II. Tax rates should be further reduced and a further increase in tax collections can be expected on doing so.Correct
Incorrect
Unattempted
-
Question 193 of 200
193. Question
1 pointsCategory: Logical ReasoningStatement:
An unacceptable number of children die during the first year of their lives. The high incidence of infant deaths is a major cause for concern for the health ministry
Courses of Action
I. All government hospitals should be privatized to improve health care facilities.
II. Governments should commit higher levels of their budget to health servicesCorrect
Incorrect
Unattempted
-
Question 194 of 200
194. Question
1 pointsCategory: Logical ReasoningStatement:
The cream of Pakistan`s cricket team is likely to retire in the next three years leaving a vacuum which the Pakistan cricket team is going to struggle to overcome.Courses of Action
I. The PCB should start to induct youngsters into the team and start to give them exposure to pressure situations.
II. There should be a rotation policy adopted for senior players in order to prolong their careers and keep them injury free.Correct
Incorrect
Unattempted
-
Question 195 of 200
195. Question
1 pointsCategory: Logical ReasoningStatement: Studies of global warming show that the earth could be hotter by at least 6 degree C in the next 100 years, thanks to huge greenhouse gas emission.
Courses of Action
I. Since greenhouse gases are responsible for global warming, steps should be taken to control their emission immediately.
II. All new industries should be immediately stopped from starting to control environmental damage.Correct
Incorrect
Unattempted
-
Question 196 of 200
196. Question
1 pointsCategory: Logical ReasoningStatement:
“New students of our college get frightened by ragging. The ragging prevalent in our college is also creating a bad name for our college”
Courses of Action
I. The college authorities take stringent action against those who are involved.
II. A strict anti ragging law should be passed to control ragging in our collegeCorrect
Incorrect
Unattempted
-
Question 197 of 200
197. Question
1 pointsCategory: Logical ReasoningStatement:
The presence of Mafiosi in the education system of Sindh has increased drastically.
Courses of Action
I. There should be a special taskforce constituted to clean the system of its ills.
II. The Sindh government should resign immediately.Correct
Incorrect
Unattempted
-
Question 198 of 200
198. Question
1 pointsCategory: Logical ReasoningStatement:
The Mobilink is playing dirty tricks with its competitor Telenor.
Courses of Action
I. Telenor should also do the same
II. Telenor should decrease the tariff rate of phone calls.Correct
Incorrect
Unattempted
-
Question 199 of 200
199. Question
1 pointsCategory: Logical ReasoningStatement:
There is a proposal for the Sindh government to clear the slum areas in Karachi for beautification and economic development.
Courses of Action
I. The Sindh Government should compensate the affected persons with reasonable amount.
II. The Sindh Government should stop beautification and economic development work immediately.Correct
Incorrect
Unattempted
-
Question 200 of 200
200. Question
1 pointsCategory: Logical ReasoningRice : Cook :: Fish : ?
Correct
Incorrect
Unattempted
Leaderboard: FLP-06
| Pos. | Name | Entered on | Points | Result |
|---|---|---|---|---|
| Table is loading | ||||
| No data available | ||||